|
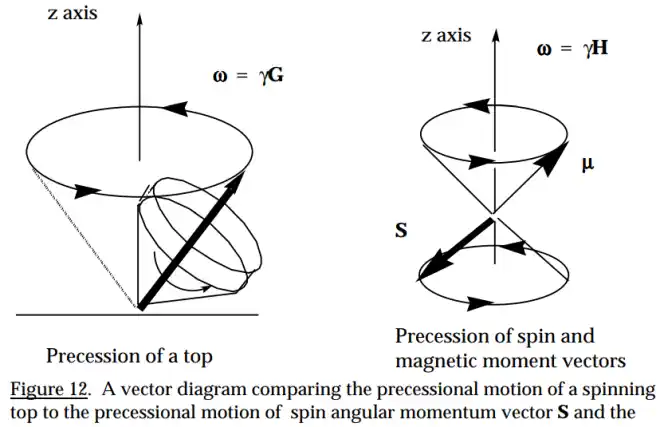
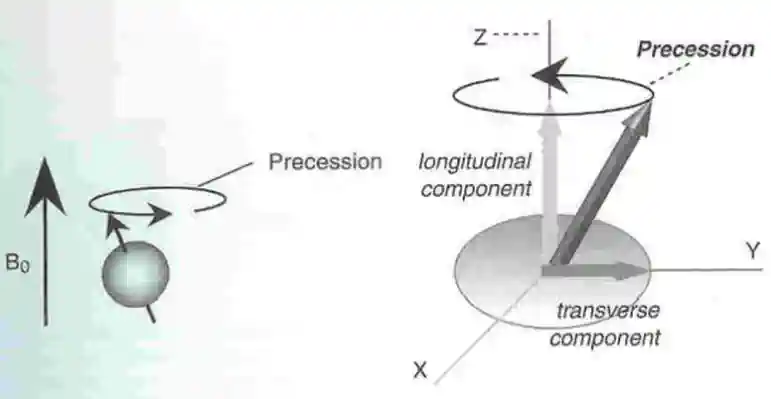
These days, in 99.99% of all textbooks and websites, when people refer to a charged particle's "spin axis", they are referring to the quantum definition of "spin axis" which is actually the precession axis of the charged particle's real spin axis. As exemplified in the diagram to the right, now days, most people don't even include the actual spin axis. They only show the precession axis and call it the spin axis.
In quantum theory, this "spin axis" by convention is synonymous with the "Z axis". When measured, this spin axis orienation is either "UP" or "DOWN", but keep in mind that it is the precession axis that is either UP or DOWN.
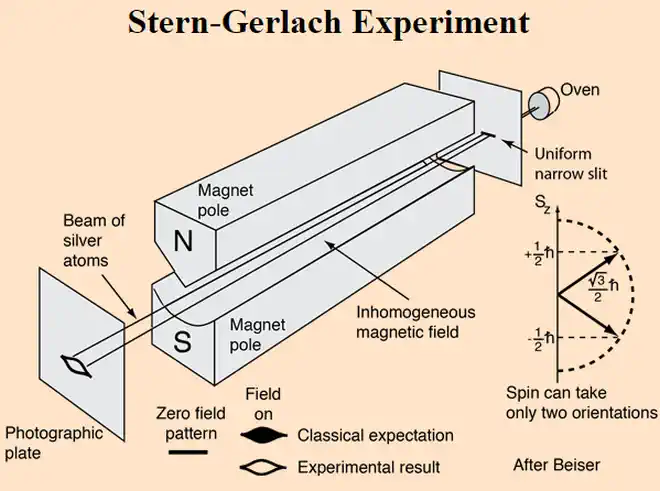
The only directions possible are just UP or DOWN because the measurement device establishes these as the only 2 options. No matter what direction the real classical spin axis might be oriented beforehand, as soon as an external magnetic field is applied (as part of taking a measurement) the laws of classical physics require the real spin axis to start precessing about the direction of the applied external magnetic field. But charged particles will be precessing already due to the influence of the sea of standing waves as described on this website's homepage.
It is the influence of the sea of standing waves that force the electron to only certain precession angles and so the quantized effect.
Regarding the subject of which is the "real" spin axis, things get even more confusing when discussing the permeability of magnetic materials. The "real" permeability u' refers to the permeability inline with the precession axes of the spins responsible for the material's magnetic properties, but the permeability u" associated with the precessing orientations of the "real" spin axes within the material is called its "imaginary" permeability.
|
|
Quantum Electron Spin
Precessional Vectors
Precession Axis
Stern Gerlach Experiment
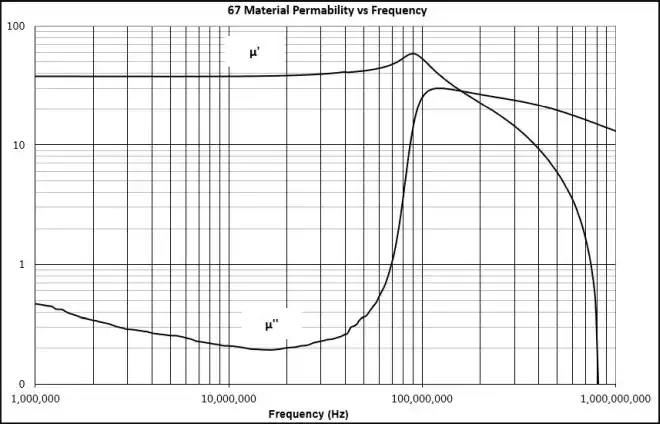
Real & Imaginary Permeability vs. Frequency
|
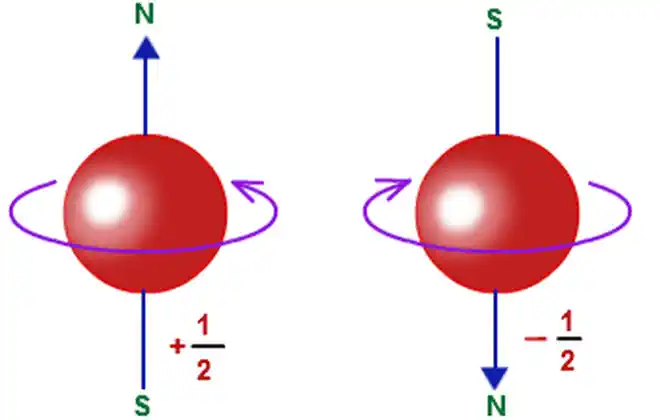
Spin UP, Spin Down:
In Quantum theory, the axis of gyroscopic precession of an electron is considered to be its spin axis. Spin Up just means that when you measure to see which way an electron's axis of precession is oriented, it is detected to have the magnetic North of its axis of precession oriented facing magnetic South of the measuring device (aligned with). Spin Down simply means when you measure to see which way an electron's axis of precession is oriented, it is detected to have the magnetic North of its axis of precession oriented facing magnetic North of the measuring device, (aligned against). The spin Down orientation with respect to an external magnetic field is considered a higher energy state while a spin Up orientation is a lower energy state. When there is no measuring equipment involved, Spin UP and Spin DOWN simply mean that the spin axes of 2 particles are oriented in opposite directions.
By convention, a "Spin Up" electron orientation relative to an external magnetic field is aligned with the external magnetic field and this is a lower energy state than when it is "Spin Down" aligned against an external field. This means that if a measuring device has its external magnetic field oriented with its North at the top and its South at the bottom then an electron's axis of precession has its North pointed down to aligned with the external field when it is in a Spin Up state. Spin UP and Spin Down do not refer to physical orientation necessarily. In other words, an external magnetic field could be oriented horizontally and an electron could be oriented horizontally to align with or against that field and still that electron is considered to be in a Spin Up or Spin Down state, depending on whether it is aligned with or against the external magnetic field.
UPDATE: I no longer know what the "convention" is because various sources are showing opposing diagrams. Here are 3 examples:
Here the website's diagram shows the spin UP electron's magnetic flux aligned against the external magnetic flux direction even while the diagram's text says it is aligned with. The source magnet could just as easily be a C shaped magnet and so this diagram is very confusing:
principles-of-general-chemistry-v1.0/section_10/a099e02893dbe7684308e61389fbd611.jpg
Here the website's diagram shows the spin UP electron's magnetic flux aligned with the external magnetic field but this is opposite to the first diagram:
Fillus_spin_1.jpg&f=1
Here the website's diagram does not show the orientation or even the existence of an external magnetic field, which makes the diagram ambiguous for anything other than showing that UP and DOWN are in opposite direction between 2 electrons:
http://images.tutorvista.com/cms/images/83/electron-spin.PNG
Never-the-less, when an electron is aligned against an external magnetic field, this is a higher energy state because there is potential energy stored between an external field and an electron with its axis of precession (Z axis) and associated magnetic field oriented against the external field. Work can be performed on the electron to flip its spin axis over to align with an external magnetic field either by making the external field strong enough to overcome the exchange interaction keeping it propped up against the external field or by stimulating it (with an electromagnetic signal at its frequency of precession) to flip over and align with the external field. When it moves into alignment it radiates its own electromagnetic signal (which is usually lost as heat in the magnetic material) and now it has lost the stored potential energy and is in a lower energy state.
Electron Spin and the Pauli Exclusion Principle:
hyperphysics.phy-astr.gsu.edu/hbase/pauli.html
www.britannica.com/nobel/micro/455_33.html
The statement at:
www.lns.cornell.edu/spr/2003-12/msg0057078.html
is wrong regarding no relation to the electromagnetic force. Two (paired) electrons in the same orbital are communicating electromagnetically so each knows the state of the other so that they remain in opposite states, i.e. one spin up and the other spin down. Many scientists get so caught up in the math that they lose sight of the real world processes that must be occurring to explain the characteristics of matter that are described by the math. The wave functions of the electrons are actually the result of their electromagnetic interaction with each other and with the sea of electromagnetic standing waves among all matter. They can't remain in opposite states (so as to satisfy the Pauli Exclusion Principle) unless they always know each others' state through continuous electromagnetic interaction. Each electron has both spin and gyroscopic precession. The gyroscopic precession causes each electron to radiate electromagnetic waves that apply forces on the other keeping its spin orientation opposite of the other. These exchanged electromagnetic waves are synchronized with the sea of standing waves among all electrons that are also radiating and absorbing due to their precessional motions.
Compensated and Uncompensated:
Paired electrons each have a magnetic field through their respective spin axes. A portion of this magnetic field is aligned along each electron's "Z axis" and a portion of the magnetic field continuously precesses about the electron's "Z axis". The path of magnetic flux lines tends to be from the magnetic North of 1 electron to the magnetic South of the other. The effect of this is that no net magnetic field is detectable from an electron pair outside the atom containing the pair. The magnetic field of each electron of a pair is said to be compensated by the magnetic field of the other electron.
An unpaired electron in an orbital of an atom also has a magnetic field through its axis of precession but since its magnetic field is not compensated by another electron with opposite spin axis orientation its magnetic field extends beyond the atom and interacts with the magnetic fields of unpaired electrons of neighboring atoms.
In some magnetic materials, like iron, an electron can be shared between atoms such that it is unpaired so its magnetic field is uncompensated and so its magnetic field can interact with the magnetic fields of neighboring unpaired electrons and with external magnetic fields. In other magnetic materials an electron can be paired but also shared between atoms in such a way as to tilt its axis of precession (canted) so that its spin is not completely compensated by its "paired" electron. The uncompensated portion of its magnetic field will interact with other partially uncompensated electrons of neighboring atoms. Actually, there are many ways that particular electron spins can be only partially compensated depending on the molecular structure and this gives rise to magnetic properties in a material.
Read more about compensated and uncompensated:
groups.google.com/groups?q=compensated+uncompensated+spin
Read more about magnetic materials:
www.geo.umn.edu/orgs/irm/hg2m/hg2m_b/hg2m_b.html#ferrimagnetism
magician.ucsd.edu/~ltauxe/sio247/lectures/lecture4.pdf
www.egs.mmu.ac.uk/users/shoon/pers_page/envmagn_tables_anal/Intro-Env-Magn.pdf
Exchange and Super Exchange Interaction:
All electrons naturally tend to align with an external magnetic field when free to do so but, when there is electromagnetic interaction between paired electrons then the pair is not susceptible to outside magnetic fields. Also, for uncompensated electrons, when there is electromagnetic interaction among neighboring uncompensated electrons, this interaction applies electromagnetic forces that can keep electrons facing against an external field. This electromagnetic interaction comes partially from Coulomb forces but mainly from electromagnetic energy radiated and absorbed due to the precessions of the electrons' magnetic dipoles. This interaction is call Exchange Interaction and Super Exchange Interaction. Quantum physicists call Exchange Interaction an entirely quantum mechanical effect but this is not true, only they fail to recognize the classical electrodynamic nature of these interactions. When electrons precess, they radiate and absorb electromagnetic energy from their magnetic dipoles' sweeping around through space. This energy when absorbed by an electron can apply more force keeping it "propped up" against an external magnetic field than an external magnetic field can apply to make it flip over and align with the external field.
Read more about these interactions at: https://en.wikipedia.org/wiki/Exchange_interaction
The Einstein, Podolsky and Rosen (EPR) Paradox:
http://www.npl.washington.edu/AV/altvw37.html
The Einstein, Podolsky and Rosen (EPR) paradox goes away after realizing that particles are in a particular state even before we attempt to measure that state. However, the act of measuring does affect that state. For example, when analyzing an electron and positron that initially have spin 1/2 and -1/2 with respect to each other, the electron can have any random orientation of its spin axis and when you measure it, it will precess about the applied external magnetic field of the measuring device. This causes the "Z axis" to be exactly aligned with or against the external field since the "Z axis" is actually the axis of precession. The orientation of its axis of precession will always be allied exactly with or against the applied external magnetic field since the electron will naturally start precessing around the direction of an external magnetic field. Then if you measure the orientation of its previously entangled positron you will find that it also precesses about the external magnetic field of a measuring device and its axis of precession will be the opposite orientation of the electron solely because its previously random orientation was already exactly opposite that of the electron it was entangled with before.
The Pauli Exclusion Principle and Compensated Spins |

